Dental Implant Mastery: Key Strategies for Optimal Osseointegration
Dental implants represent one of the most transformative advancements in modern dentistry. Since the introduction of titanium implants for intra-oral use in the late 1950s, implantology has evolved into a highly effective solution for oral rehabilitation in partially or fully edentulous patients. The groundbreaking concept of osseointegration, introduced by Dr. Brånemark and colleagues over 45 years ago, revolutionized the field, sparking extensive research into implant shapes, materials, and designs.

Today, the dental implant market boasts approximately 1,300 distinct systems, each varying in shape, size, material composition, thread design, implant-abutment connections, surface topography, chemistry, wettability, and modifications. With such a vast array of options, selecting the right dental implant can be a daunting task for both dental professionals and patients. How can you ensure that the chosen implant delivers exceptional clinical performance, cost-effectiveness, and long-term value?
Here are the key factors to consider when selecting the ideal dental implant:
-
Clinical Research and Long-Term Success: Prioritize brands with a strong track record of clinical success and robust research supporting their efficacy.
-
Compatibility and Versatility: Choose a brand that offers a wide range of implant designs, abutment connections, and prosthetic components to accommodate diverse clinical cases.
-
Ease of Use: Opt for brands that provide user-friendly surgical kits, comprehensive training, and reliable support to streamline the implantation process.
-
Aesthetic Outcomes: Select implants that promote healthy soft tissue integration and offer customizable aesthetic solutions.
-
Balance Between Cost & Quality: Focus on long-term value rather than initial cost. Some brands offer cost-effective options without compromising quality.
-
Warranty and Global Availability: Ensure the brand provides dependable warranties and global access to replacement parts and components.
-
Futureproofing: Choose brands that embrace innovation and integrate seamlessly with digital workflows to keep your practice at the forefront of technology.
DESS® CONICAL BLT: A Case Study in Excellence
DESS® Dental, serving over 40 countries, provides a comprehensive selection of dental abutments and prosthetic solutions fully compatible with leading implant systems. Expanding its product portfolio, DESS® has introduced two new implant systems, including the DESS® CONICAL BLT—a bone-level tapered implant precisely designed for seamless integration with the Straumann® BL system.
This implant exemplifies the key criteria for optimal implant selection:
-
Preservation of Crestal Bone: Maintains crestal bone levels and minimizes micro-gaps.
-
Healing Flexibility: Supports both transmucosal and submucosal healing protocols.
-
Enhanced Stability: Features a 15-degree internal conical connection with four internal grooves to reduce screw loosening.
-
Advanced Surface Technology: Incorporates DESS® Osseointegration Surface Technology (OST), adhering to SLA standards for superior osseointegration.
Click here to learn more about Conical BLT Dental Implants
Pilot Study: Comparing DESS® with Leading Implant Systems
After over a year of successful use, the DESS® CONICAL BLT implant has demonstrated exceptional performance. To further validate its efficacy, we led a dedicated team to conduct a pilot study comparing DESS® implants with three premium brands: Straumann®, Nobel® Biocare, and Astra Tech Implant System™ EV.
This initial study will provide valuable preliminary data to evaluate the performance of these implant systems, laying the groundwork for future, more extensive research.
ABSTRACT
Purpose
This study aims to compare marginal bone changes around DESS® (Osseointegration Surface Technology) implants with those around three leading implant systems—Straumann® (SLA), Astra Tech™ (OsseoSpeed), and Nobel Biocare® (TiUnite)—using Cone Beam Computed Tomography (CBCT) at a 3-month follow-up after implant placement.
Materials and Methods
Study Design
A non-randomized prospective cohort study was conducted with 40 patients from our clinic. Each patient received one of four implant types in the mandible: DESS®, Straumann®, Astra Tech®, or Nobel Biocare®.
Grouping
Patients were divided into four groups of 10, with each group assigned to one implant system. Implants were placed in the mandible following a standard delayed implant placement protocol. Baseline CBCT imaging was performed immediately after implant placement to measure initial marginal bone levels. A follow-up CBCT scan was conducted 3 months post-surgery to assess changes in marginal bone levels. Marginal bone loss (MBL) was calculated as the difference between baseline and 3-month measurements. Two independent examiners, trained in CBCT analysis, performed the measurements to ensure accuracy and reliability.
Results
The average marginal bone loss across all implants was approximately 0.5 mm, with minor variations between systems. All groups demonstrated significant bone loss (p < 0.05) from baseline to 3 months, though differences between groups were minimal. A one-way ANOVA was conducted to compare mean MBL among DESS®, Straumann®, Astra Tech®, and Nobel Biocare® implants.
The analysis revealed no statistically significant differences in marginal bone loss between the four groups (p > 0.05), indicating comparable performance across all systems. The intra-class correlation coefficient (ICC) for the two examiners was 0.95, reflecting excellent reliability in bone level measurements.
Conclusion
This preliminary study found no significant differences in marginal bone loss among the four implant systems (DESS®, Straumann®, Astra Tech®, or Nobel® Biocare) after three months. The decision to use one implant system over another may instead depend on other clinical considerations, such as cost, prosthetic compatibility, or patient preference. Further research with larger sample sizes is recommended to identify any subtle differences between these implant systems.
-
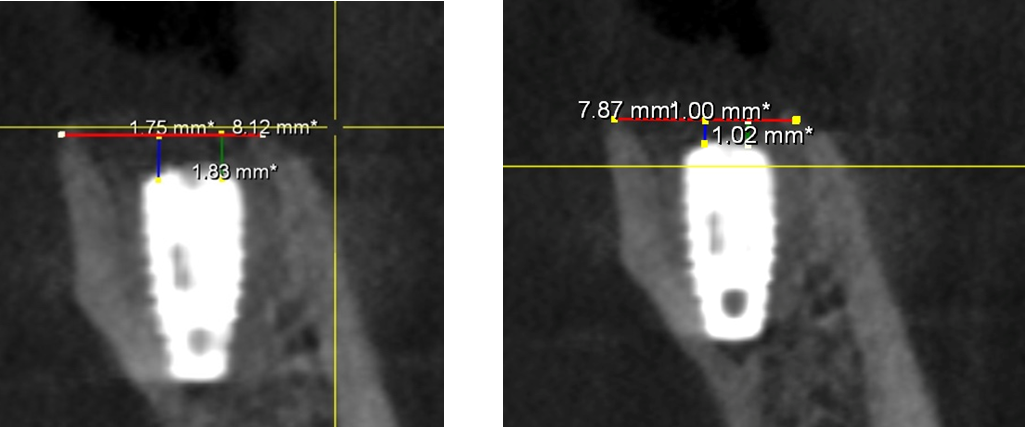
Figure 1 (A): A CBCT image captured immediately after implant placement, illustrating the initial condition of the crestal bone level.
-
Figure 2 (B): A CBCT image taken three months post-implant placement, showing the measurement of the crestal bone level after this period.
Figure: Marginal bone loss after 3 months shows comparable performance across implant systems, with slight variations in mean bone loss.
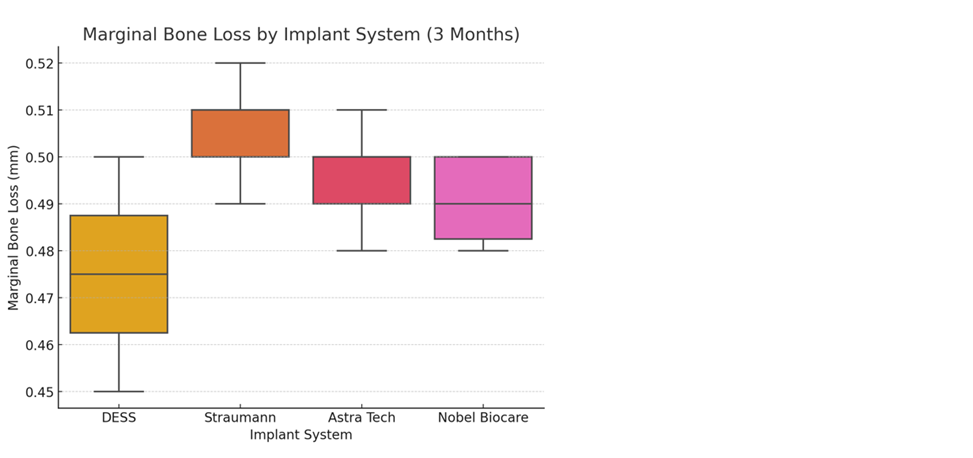
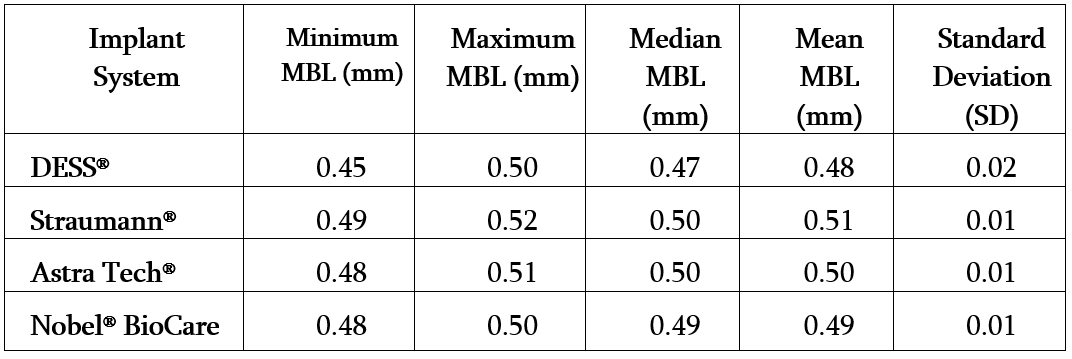
Table: Summary of Marginal Bone Loss (MBL) Distribution by Implant System
In summary, the DESS® CONICAL BLT implant stands out as a reliable and versatile option, offering comparable performance to leading premium brands. Its innovative design, combined with proven clinical outcomes, makes it a strong contender for dental professionals seeking optimal osseointegration and patient satisfaction.



